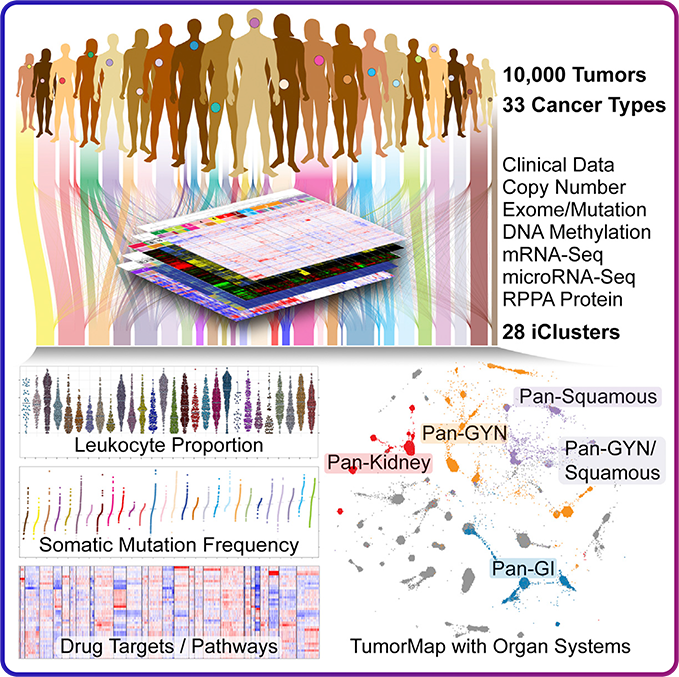
Cancer Precision Therapy
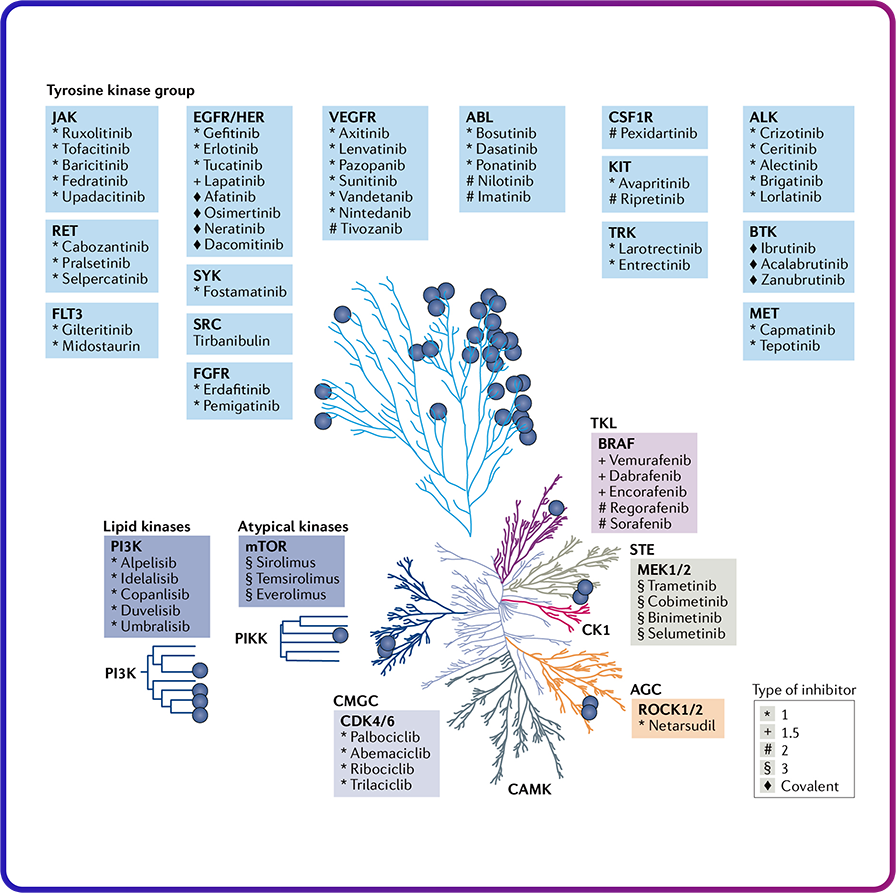
Kinase Inhibitors
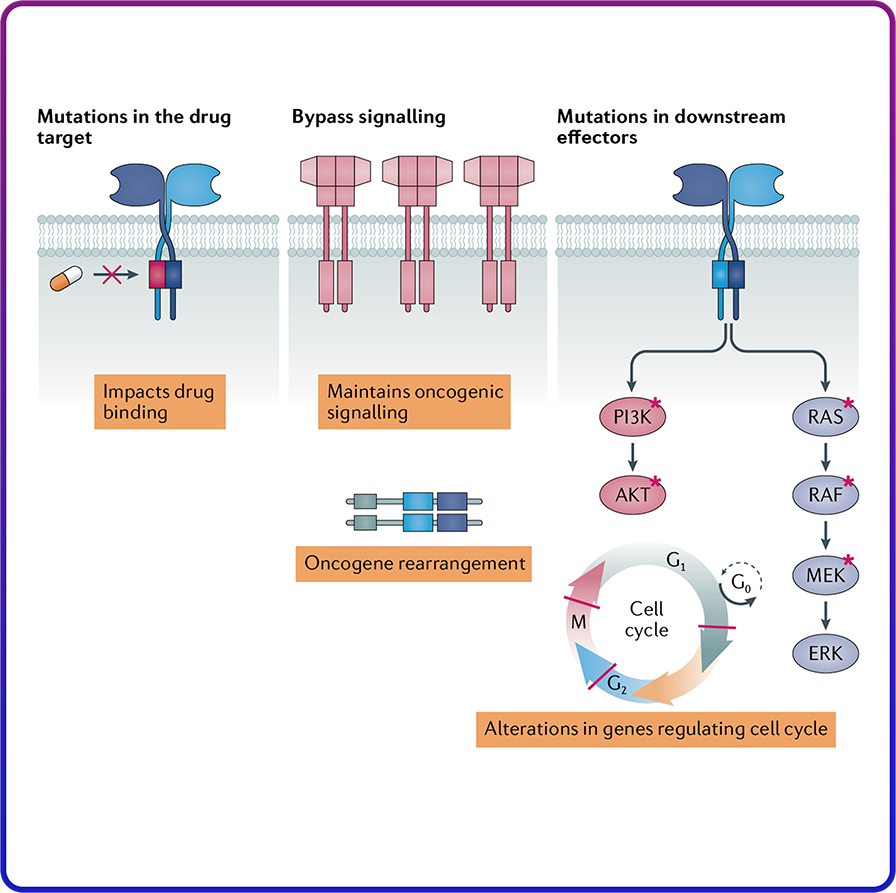
Mutation Induced Resistance
Based on APS’s independent Integrated Computational Chemical Biology (ICCB) drug development platform, APS focuses on a deep understanding of cancer genetics, protein structure and function, synthetic medicinal chemistry, experiment and Computation integrated verification technology. We are seeking to develop additional therapeutic areas and next-generation precision therapies that target genetic drivers of cancer.

A Phase 1,multi-center clinical trial of APS03118 in RET positive NSCLC, MTC and other solid tumor patients is being conducted in China.

Address
Office: San Diego,US
Office: Building 10-1, No.2 Jingyuan North Street, BDA, Beijing, China
Tel
+86-10-67860673
