
News
Focus on the latest innovative drug technology, deliver the therapeutic hopes for patients


APS03118 is an innovative drug developed by APS for unlimited cancers with global independent intellectual property rights. Potential indications include non-small cell lung cancer(NSCLC), thyroid cancer, breast cancer, colorectal cancer and other metastatic solid tumors with RET aberrances.
RET aberrances contain fusions and mutations that lead to over-activation of RET signaling pathways and uncontrolled cell growth. As RET oncogene is present in a variety of cancers,it has become an important target of "unlimited cancer" therapies. Tumors with RET alterations primarily rely on abnormal activation of this kinase to promote proliferation and growth, therefore RET positive tumors are sensitive to RET inhibitors.
Although RET oncogene is closely related to various of cancers, only selpercatinib and pralsetinib were approved recently as the selective RET inhibitors. Selective RET inhibitors are effective in RET positive patients but followed by acquired drug resistance and progress disease. On-target resistances include the acquisition of solvent front mutations (SFMs) RET G810 R/S/C that have been identified as a predominant mechanism to both selpercatinib and pralsetinib. The roof RET L730I/M, gate keeper RET V804M/L/E, and hinge RET Y806 mutations are also important resistance mutations to current MKI and selective RET inhibitors. Therefore, a next-generation RET inhibitor should potently against SFMs and a broad range of other mutations.
APS03118 is the highly selective and potent next-generation RET inhibitor
• APS03118 was highly selective against a panel of 468 kinases at 100 nM compound concentration (performed at Eurofins).
• No concerning off-target finding in Eurofins Safety47 panel at the 10 μM compound concentration.
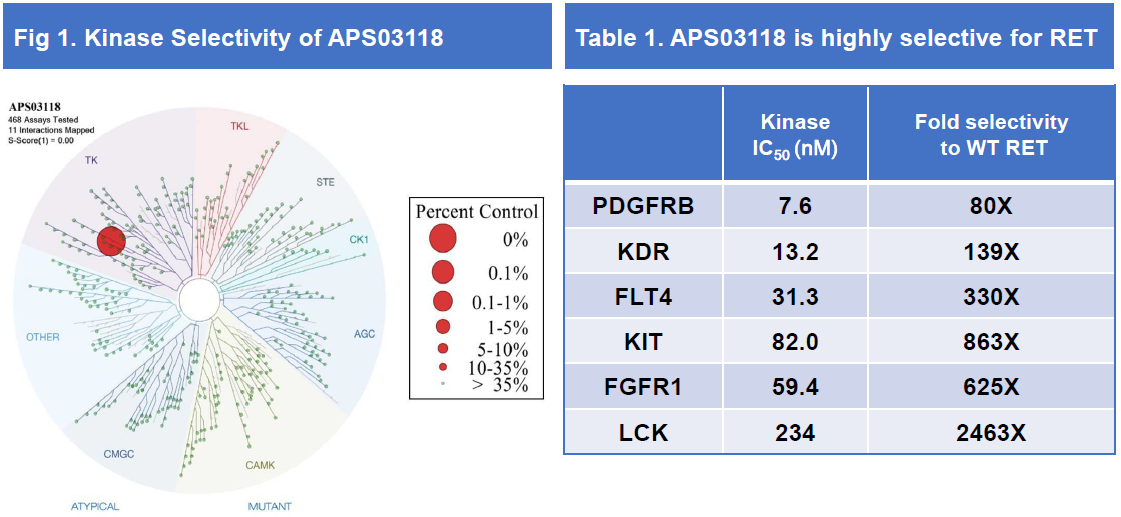
APS03118 is potent against RET alterations in vitro
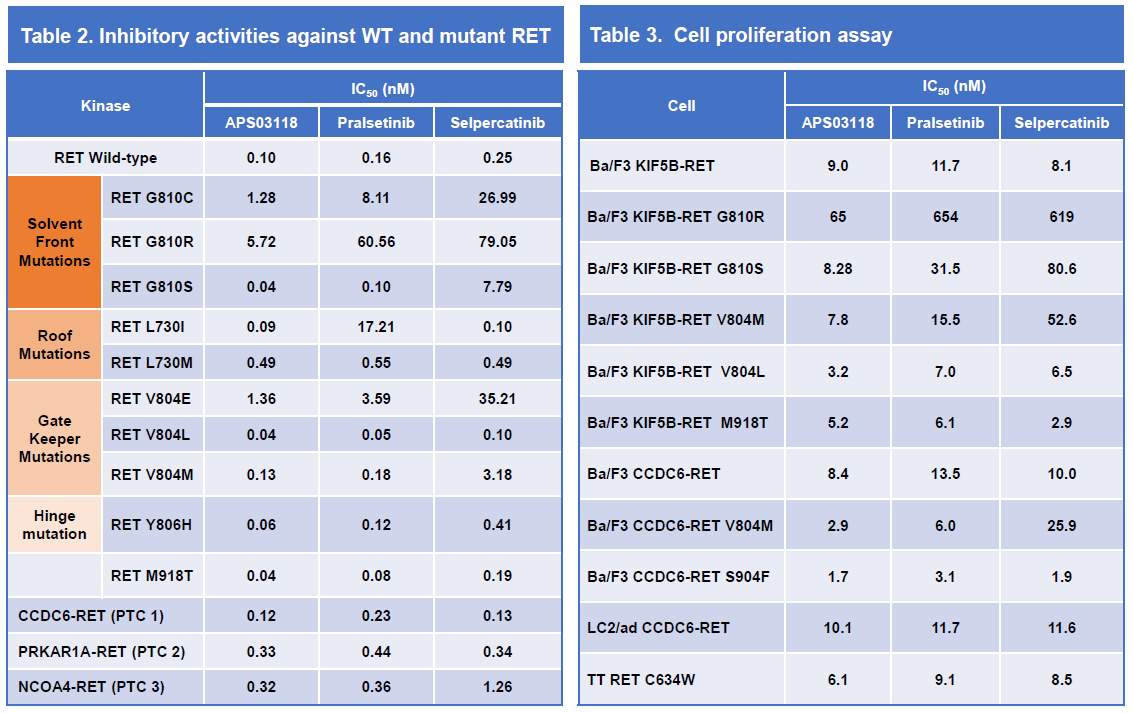
• APS03118 is potent against wild-type (WT) and a board range of mutant RET in comparison with selpercatinib and pralsetinib in the enzymatic and cell-based assays, especially the SFMs RET G810 R/S/C, gate keeper RET V804M/L/E, roof RET L730I/M and hinge RET Y806 mutations that induced the resistance to RET inhibitors.
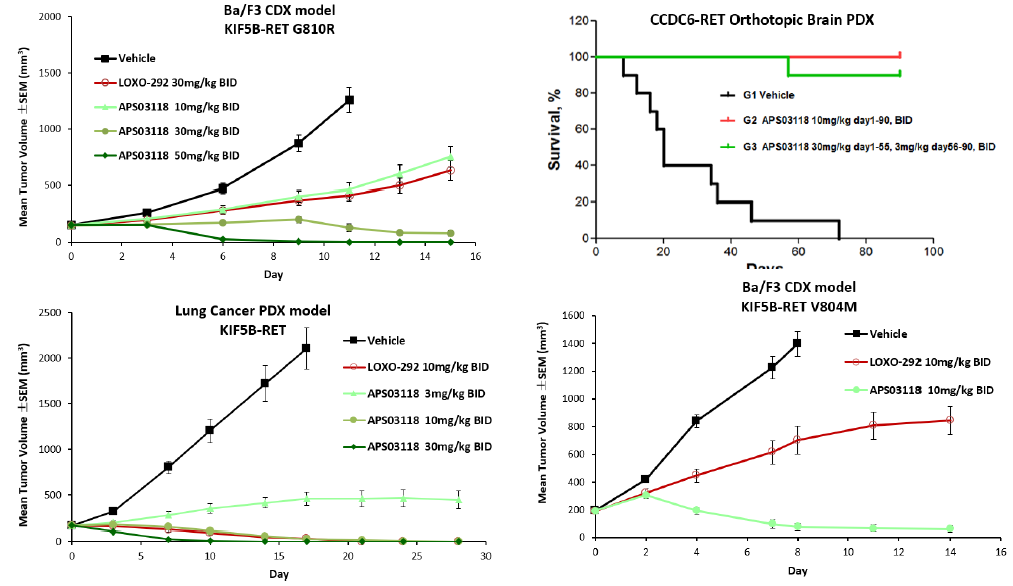
APS03118 shows potent antitumor efficacy in xenograft mouse tumor models
• APS03118 is highly active in xenograft tumor models including KIF5B-RET PDX, KIF5B-RET V804M CDX and KIF5B-RET G810R CDX models.
• Tumors completely subsided in orthotopic brain PDX model harboring CCDC6-RET with a 100% survival rate in APS03118 treated group.
RET is closely related to the occurrence of a variety of human tumors. Lung cancer is the malignant tumor with the highest morbidity and mortality in China, and NSCLC is the most common type of lung cancer. NSCLC patients with RET gene alterations are not uncommon in clinic. In addition, the incidence of brain metastasis is high in lung cancer patients, which is closely related to the positive RET fusion with the cumulative incidence of more than 60% in 24 months. APS03118 may provide a better therapeutic option for NSCLC and thyroid cancer induced by RET aberrances.
Dr. Jun Zhong, vice president of R&D of APS, stated: "APS03118 has proved its great potential in the treatment of cancers with RET aberrances in preclinical studies. By participating the AACR meeting, We hope the RET-targeted therapy would attract more attention, and provide optimized options for the patients with acquired resistance to the first-generation selective RET inhibitors in response to the global unmet medical needs in this field."
Investigational New Drug (IND) application of APS03118 was approved by FDA early this year, and APS03118 was also granted the Fast Track Designation by FDA. APS03118 released key preclinical data on AACR meeting would add more confidence to ongoing global clinical trial.
Applied Pharmaceutical Science, Inc. is a biopharmaceutical high-tech company focused on innovative cancer precision therapy and tumor-driven gene drug development through in-depth exploration of cancer-causing driver genes, protein structure and function, and synthetic medicinal chemistry. APS aims to explore the life sciences, innovate precision cancer therapy, and seek new hope for cancer patients worldwide with breakthrough tumor precision therapies.
Please contact us for further communication about company and products:
info@apspharm.com
The news cover image comes from the official website of AACR conference.

Address
Office: San Diego,US
Office: Building 10-1, No.2 Jingyuan North Street, BDA, Beijing, China
Tel
+86-10-67860673
